
SHARE THIS ARTICLE:
FDA Approves Tests to Screen Blood Supply for Babesia
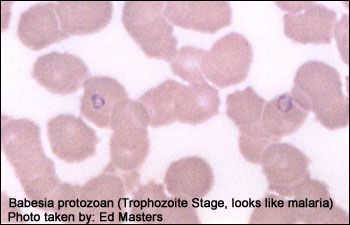 The U.S. Food and Drug Administration (FDA) has approved the first tests to screen human plasma and whole blood samples for the Babesia parasite species, Babesia Microti (B. Microti), the main species that causes infection in the United States. Babesia parasites are transmitted by Ixodes scapularis ticks, also known as blacklegged or deer ticks but can also be transmitted by transfusion of blood or blood components collected from an infected donor. These new tests will be performed on human donor samples, which includes volunteer donors of whole blood and blood components, as well as living organ and tissue donors.
The U.S. Food and Drug Administration (FDA) has approved the first tests to screen human plasma and whole blood samples for the Babesia parasite species, Babesia Microti (B. Microti), the main species that causes infection in the United States. Babesia parasites are transmitted by Ixodes scapularis ticks, also known as blacklegged or deer ticks but can also be transmitted by transfusion of blood or blood components collected from an infected donor. These new tests will be performed on human donor samples, which includes volunteer donors of whole blood and blood components, as well as living organ and tissue donors.
The approval was granted to Oxford Immunotec, Inc. for the Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) to detect antibodies to B. Microti in human plasma samples, and the Imugen Babesia microti Nucleic Acid Test (NAT) to detect B. Microti DNA in human whole blood samples. Both tests can only be performed at the Norwood, Massachusetts facility of Oxford Immunotec, Inc.
Studies performed by the manufacturer and previous investigational use of Babesia donor testing in endemic areas have shown these tests are effective in screening donors for B. microti infection.
As noted in the FDA news release: “The U.S. Centers for Disease Control and Prevention (CDC) warns that for certain people, especially those with a weak immune system, it [B.microti infection/babesiosis] can be a severe, life-threatening disease and that while bloodborne transmission of babesiosis is thought to be uncommon, it is the most frequently reported transfusion-transmitted parasitic infection in the U.S. and remains an important concern.”
Click here for FDA News Release





